- Valence Electrons Calculator
- Core Electrons Selenium
- Selenium Valence Electron Count
- Selenium Atom Valence Electrons
- Selenium Dihydride Total Valence Electrons
Learning Objective
Mar 22, 2019 We know that the atomic number of selenium is 34.So selenium has 34 protons and 34 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1. Step-3: Now write the electron configuration of selenium. Se (34)=1s²2s²2p⁶3s²3p⁶4s²3d¹º4p⁴. A selenium atom has six valence electrons. Do you think it will lose six electrons or gain two electrons to obtain an octet in its outermost electron shell? An iodine atom has seven valence electrons. Do you think it will lose seven electrons or gain one electron to obtain an octet in its outermost electron shell? Electrons have a specific form of distribution (or configuration) in every atom, even Selenium. Some are hard to memorise (or predict), so what is the electron configuration of an atom of Se? In the case of Selenium the abbreviated electron configuration is Ar 3d10 4s2 4p4. Nevertheless, check the complete configuration and other interesting. The number of electrons in per shell of selenium atom is 2, 8, 18, 6. So, there are six electrons in the valence shell of the selenium. The electronic configuration of selenium atom is 4s 2 3d 10 4p 4.

- Draw a Lewis electron dot diagram for an atom or a monatomic ion.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (The order in which the positions are used does not matter.) For example, the Lewis electron dot diagram for hydrogen is simply
[mathbf{H}mathbf{cdot}]
Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:
[mathbf{dot{H}}; ; or; mathbf{cdot}mathbf{H}; ; ; or; ; ; mathbf{underset{.}H}]
The electron dot diagram for helium, with two valence electrons, is as follows:
[mathbf{He}mathbf{:}]
By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later. The next atom, lithium, has an electron configuration of 1s22s1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used:

[mathbf{Li}mathbf{cdot}]
Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of helium:
Valence Electrons Calculator
[mathbf{Be}mathbf{:}]
The next atom is boron. Its valence electron shell is 2s22p1, so it has three valence electrons. The third electron will go on another side of the symbol:
[mathbf{dot{B}}mathbf{:}]
Again, it does not matter on which sides of the symbol the electron dots are positioned.
For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. As usual, we will draw two dots together on one side, to represent the 2s electrons. However, conventionally, we draw the dots for the two p electrons on different sides. As such, the electron dot diagram for carbon is as follows:
[mathbf{cdot dot{C}}mathbf{:}]
With N, which has three p electrons, we put a single dot on each of the three remaining sides:
[mathbf{cdot}mathbf{dot{underset{.}N}}mathbf{:}]
For oxygen, which has four p electrons, we now have to start doubling up on the dots on one other side of the symbol. When doubling up electrons, make sure that each side has no more than two electrons.
[mathbf{cdot}mathbf{ddot{underset{.}O}}mathbf{:}]
Fluorine and neon have seven and eight dots, respectively:
[mathbf{:}mathbf{ddot{underset{.}F}}mathbf{:}]
[mathbf{:}mathbf{ddot{underset{.: .}Ne}}mathbf{:}]
With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell, the n = 3 shell. By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
Example (PageIndex{1}): Lewis Dot Diagrams
What is the Lewis electron dot diagram for each element?
- aluminum
- selenium
Solution
- The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons:
[dot{Al:} nonumber]
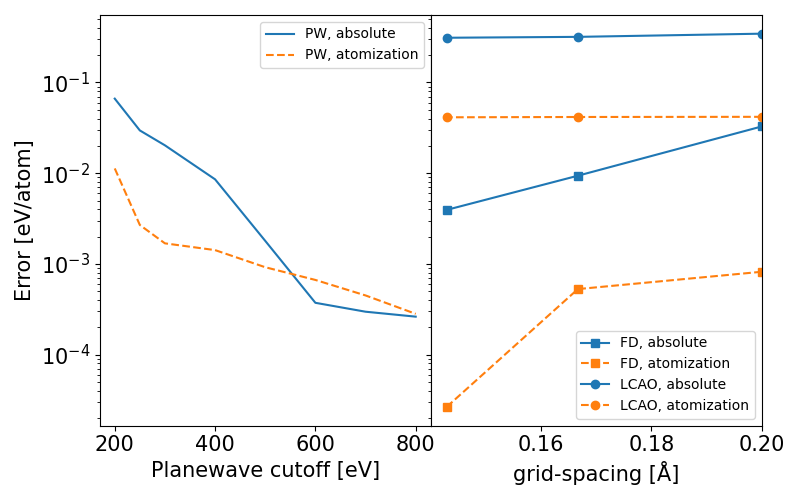
- The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:
[mathbf{cdot }mathbf{dot{underset{.: .}Se}}mathbf{:} nonumber]
Exercise (PageIndex{1})
What is the Lewis electron dot diagram for each element?
- phosphorus
- argon
[mathbf{cdot }mathbf{dot{underset{.}P}}mathbf{:} nonumber]
[mathbf{:}mathbf{ddot{underset{., .}Ar}}mathbf{:} nonumber]
Summary
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
3.1 Two Types of Bonding
Learning Objectives
- Define the octet rule.
- Describe how ionic bonds are formed.
Atoms can join together by forming a chemical bondA very strong attraction between two atoms., which is a very strong attraction between two atoms. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart.
What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? A clue comes by considering the noble gas elements, the rightmost column of the periodic table. These elements—helium, neon, argon, krypton, xenon, and radon—do not form compounds very easily, which suggests that they are especially stable as lone atoms. What else do the noble gas elements have in common? Except for helium, they all have eight valence electrons. Chemists have concluded that atoms are especially stable if they have eight electrons in their outermost shell. This useful rule of thumb is called the octet ruleThe idea that atoms tend to have eight electrons in their valence shell., and it is a key to understanding why compounds form.
Note
Of the noble gases, only krypton, xenon, and radon have been found to make compounds.
There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. One way is the transfer of electrons between two atoms until all atoms have octets. Because some atoms will lose electrons and some atoms will gain electrons, there is no overall change in the number of electrons, but individual atoms acquire a nonzero electric charge. Those that lose electrons become positively charged, and those that gain electrons become negatively charged. Charged atoms are called ionsA charged atom.. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bondsAn attraction between oppositely charged ions.. The resulting compounds are called ionic compoundsA compound formed with an ionic bond. and are the primary subject of this chapter.
The second way for an atom to obtain an octet of electrons is by sharing electrons with another atom. These shared electrons simultaneously occupy the outermost shell of more than one atom. The bond made by electron sharing is called a covalent bond. Covalent bonding and covalent compounds will be discussed in Chapter 4 'Covalent Bonding and Simple Molecular Compounds'.
Note
Despite our focus on the octet rule, we must remember that for small atoms, such as hydrogen, helium, and lithium, the first shell is, or becomes, the outermost shell and hold only two electrons. Therefore, these atoms satisfy a “duet rule” rather than the octet rule.
Example 1
A sodium atom has one valence electron. Do you think it is more likely for a sodium atom to lose one electron or gain seven electrons to obtain an octet?
Solution
Although either event is possible, a sodium atom is more likely to lose its single valence electron. When that happens, it becomes an ion with a net positive charge. This can be illustrated as follows:
| Sodium atom | Sodium ion | ||
|---|---|---|---|
| 11 protons | 11+ | 11 protons | 11+ |
| 11 electrons | 11− | 10 electrons | 10− |
| 0 overall charge | +1 overall charge | ||
Skill-Building Exercise
A fluorine atom has seven valence electrons. Do you think it is more likely for a fluorine atom to lose seven electrons or gain one electron to obtain an octet?
Concept Review Exercises
How are ionic bonds formed?
Answers
The octet rule is the concept that atoms tend to have eight electrons in their valence electron shell.
Ionic bonds are formed by the attraction between oppositely charged ions.
Key Takeaways
- Atoms have a tendency to have eight electrons in their valence shell.
- The attraction of oppositely charged ions is what makes ionic bonds.
Core Electrons Selenium
Exercises
Selenium Valence Electron Count
Why is an ionic compound unlikely to consist of two positively charged ions?
Why is an ionic compound unlikely to consist of two negatively charged ions?
A calcium atom has two valence electrons. Do you think it will lose two electrons or gain six electrons to obtain an octet in its outermost electron shell?
An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell?
A selenium atom has six valence electrons. Do you think it will lose six electrons or gain two electrons to obtain an octet in its outermost electron shell?
An iodine atom has seven valence electrons. Do you think it will lose seven electrons or gain one electron to obtain an octet in its outermost electron shell?
Selenium Atom Valence Electrons
Answers
Selenium Dihydride Total Valence Electrons
Positive charges repel each other, so an ionic compound is not likely between two positively charged ions.
It is more likely to lose two electrons.
It is more likely to gain two electrons.
